What is Basic or Cationic Dye?
The first synthetic dye, Perkin’s Mauve belongs to basic or cationic dye class. Magenta and Malachite Green are also amongst the earliest synthetic dyes. The basic dyes are so-named because they are derived from organic bases. They are also called cationic dyes as they ionise in water producing colored cations. They are capable of salt-formation as shown in Equation.
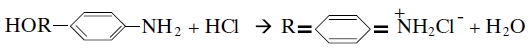
A basic dye is defined as a cationic dye characterised by its substantivity for the acidic types of acrylic fiber and for tannin mordanted cotton, which reflects the former importance of this class of dye in the dyeing of cotton, for which reactive dyes, direct dyes, vat dyes and sulphur dyes are nowadays employed. As basic dyes form colored cations of characteristically high tinctorial strength and brilliance when dissolved in water, they are referred to as cationic dyes.
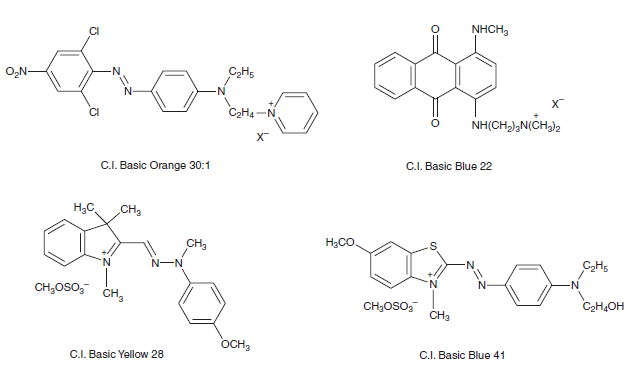
Cationic dyes used for PAN fibers are usually divided into two chemical types, namely, localised dyes (aka pendant dyes) in which the positive charge is localised on one atom (e.g. N) as exemplified by C.I. Basic Orange 30:1 and C.I. Basic Blue 22, as well as delocalised dyes, in which the positive charge is delocalised over the entire dye molecules, as exemplified by C.I. Basic Yellow 28 and C.I. Basic Blue 41.
Properties / Characteristics of Basic Dyes:
Basic Dyes are cationic soluble salts of colored bases. Basic dyes are applied to substrate with anionic character where electrostatic attractions are formed. Basic dyes are not used on cotton as the structures are neither planar nor large enough for sufficient substantivity or affinity. Basic dyes are called cationic dyes because the chromophore in basic dye molecules contains a positive charge. The basic dyes react on the basic side of the isoelectric points. Basic dyes are salts, usually chlorides, in which the dyestuff is the basic or positive radical. Basic dyes are powerful coloring agents. It’s applied to wool, silk, cotton and modified acrylic fibers. Usually acetic acid is added to the dyebath to help the take up of the dye onto the fiber. Basic dyes are also used in the coloration of paper.

Principle characteristics of basic dyes are described below:
1. Ionic nature:
The ionic nature of these dyes is cationic.
2. Shade range:
These dyes exhibit an unlimited shade range with high tinctorial strength, brightness and many colors are having fluorescent properties.
3. Solubility:
The solubility of these dyes is very good in water, in the presence of glacial acetic acid.
4. Leveling properties:
These dyes have a very high strike rate, therefore leveling is poor.
5. Exhaustion:
Cationic dyes exhaust at a variable rates, K values are used to define the exhaustion characteristics of the cationic dyes. K=1 means the fastest exhaustion, while K=5 means the slowest exhaustion. So while making the combination shades the dyes of similar K values must be used.
6. Affinity:
These dyes shows a very affinity towards wool, silk and cationic dye able acrylic, but have no affinity towards cellulosics. To dye cellulosics with basic dyes the material must be treated with suitable mordanting agents.
7. Fastness Properties:
The light fastness is poor to moderate, but wet fastness is good.
To dye cellulosics with basic dyes, the material must be treated with suitable mordanting agents.
- Work as cationic soluble salts of colored bases
- Can be applied to substrate with anionic character in areas where electrostatic attractions are formed
- Provides working as powerful coloring agents
- Can be applied to silk, wool, cotton and modified acrylic fibers
- Also used in coloration of paper
- Can offer varied shades with high tinctorial strength and brightness properties
- Having good solubility in water in presence of glacial acetic acid
- Relatively economical in usage
- Shows good brightness
Manufacturing of Basic Dyes:
1. Auromine O:
It is the most important basic yellow and is highly valued because of the extraordinarily pure tints it produces. It is made by heating a mixture of 4,4’– dimethylamino diphenylmethane, sulphur and ammonium chloride and sodium chloride (acting as diluents) to 175°C in the presence of gaseous ammonia. The resulting Auromine base is converted into its hydrochloride which is Auromine O.
2. Malachite Green:
It is prepared by condensation of two molecules of dimethylaniline with one of benzaldehyde. The leuco base, which is thus obtained, is then oxidized with oxidising agents of high electro chemical potential such as lead dioxide to give the dye salt with acid.
3. Methylene Blue:
It is made by oxidizing a mixture of dimethylaniline and p-amino-dimethyl aniline (equimolar quantities) and sodium thiosulphate with sodium dichromate and hydrochloric acid in presence of zinc chloride (to get the final dye as zinc chloride double salt).
4. New Magenta:
1kg of anhydro formaldehyde, 5 kg of toluidine hydrochloride and 1 kg of o-toluidine are heated at 170°C for 2–3 hours with 1.2 kg of o-nitrotoluine and 100 grams of iron fillings, in an enameled vessel. The unreacted o-toludine and o-nitrotoluene are removed by distillation with steam, the residue is filtered hot, and the New Fuscsin is salted out.
References:
- Textile Dyes by N. N. Mahapatra
- Handbook of Textile and Industrial Dyeing Volume 2: Applications of Dyes Edited by M. Clark
- Textile Dyes By Mansoor Iqbal
- Physico-chemical Aspects of Textile Coloration by Stephen M. Burkinshaw
- Textile Chemistry by Thomas Bechtold, Tung Pham
- Textile Engineering-An Introduction Edited by Yasir Nawab
- An Introduction to Textile Coloration: Principles and Practice By Roger H. Wardman
You may also like:
- Different Types of Dyeing Methods
- Various Classification of Dyes | Different Types of Dyes
- Natural Dyes: Dyeing Process and Environmental Impact
- Principal Classes of Synthetic Dyes and Their Characteristics
