Name of the experiment: Dyeing of Jute Fabric with Basic Dyes
Introduction:
Dyeing is a process by which a textile material is changed physically or chemically so that it looks colorful and dye is a special type organic compound which is responsible for color. Basic dyes are one of the most versatile classes of dyestuff applicable to cellulose, acrylic, modified nylon and polyester. Basic dyes are sometimes called cationic dye, because the chromophore contains a positive charge. Basic dyes are used on fibers containing acidic groups that can interact with these cationic groups. The fibers that are dye able with basic dyes contain either carboxyl or sulphonic acid groups.
Theory:
Although the dye that are suitable for cotton may also be applied for jute dyeing. But in practice, jute dyeing with these dyes has some deficiency. The fibre has a special affinity to basic dyestuff, which provides brilliant shade even on unbleached base. But the problem of these shades is lower fastness to light and water. Acid, direct and sulfur dyes are increasing fast in this order, but also give increasing dullness of shade- all at reasonable cost. Very bright and fast results are obtained with azoic and vat dyes, but their high cost confines this application.
Properties of basic dyes:
- Manufactured as salt of basic form.
- Color fastness properties vary greatly from one dye to another and what fibres they are used on.
- Wash and light fastness is very good to excellent for acrylic.
- Suitable for high brilliant shade and decomposes at boiling temperature.
Apparatus:
- Beaker
- Glass rod
- Measuring Flasks
- Bowl
- pH meter
- Burner
- Thermometer
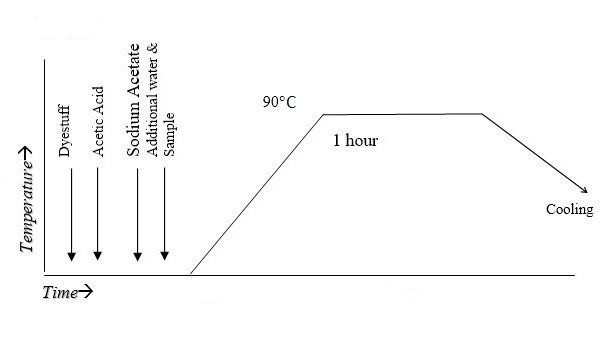
Parameters:
- pH range: 4-5
- Duration of the treatment: 40 minute
- Temperature: 90ºC
- M:L> 1:40
Recipe for dyeing:
- Dyes: 3% owf.
- Common salt: 15 g/L
- Wetting agent: 1.0g/L
- Sequestering agent: 1g/L
- Acetic acid: 2 g/L
Recipe for after treatment:
- Detergent : 2 g/L
- Time: 10 minute.
Recipe calculation:
- Weight of the fabric: 7.19 gm
- Weight of the liquid: 7.19×40gm = 287.60 gm ≈ 287.60cc
- Wetting agent: (1×287.60)/1000gm=0.29gm ≈ 0.29cc
- Sequestering agent: (1×287.60)/1000gm=0.29gm ≈ 0.29cc
- Common salt: (15×287.60)/1000gm=4.314gm
- Acetic acid: (2×287.60)/1000gm=0.58gm≈ 0.56cc
These values are calculated on the basis of the weight of the liquid.
Chemicals and its function:
| Chemicals | Functions |
| Dye (basic dye) | Main color producing agent containing chromophore group. |
| Wetting agent (T.R oil) | Reduce surface tension and interfacial tension, helps to wet out materials with liquid. |
| Common salt (NaCl) | Used to assists the diffusion and exhaustion of direct dye anionic by neutralizing the negative surface charge of cellulosic fibres |
| Sequestering agent | To deactivate the metal ion. |
| Acetic acid | To maintain the pH of the bath. |
Procedure:
- Set the dye bath with substrate at room temperature.
- Add required dyes solution into the bath with salt, acid and other bath auxiliaries
- Raise the temperature and run the dye bath for 40 minutes at 90-95ºC for complete the dyeing cycle.
- Cool down the bath temperature to 60-70ºC.
- Drop the bath and carry on the after treatment process.
After treatment process:
The dyed goods need successive hot and cold wash to remove unfixed dyes for improving colorfastness. For this process we applied only detergent. Here jet is used as detergent for 10 minutes. Finally we wash it cold water.
Result:
Dyeing is the most important process of fibre processing as well as textile goods. By this experiment we come to know about the dyeing process, especially the use of basic dyes experimentally. We also know the recipe and controlling points of using basic dye.
You may also like:
- General Dyeing Procedure of Cotton Fabric with Recipe
- What is Basic or Cationic Dye | Properties and Manufacturing of Basic Dyes
