Supercritical Fluid Dyeing: Need of the Future
Siddharth Agatrao Gaikwad
DKTE’S Textile and Engineering Institute, Ichalkaranji, India
B.Tech in Textile Chemistry
Email: gsiddharth646@gmail.com
Introduction
A dye or dyestuff is defined as a coloured organic compound or mixture that may be used to impart colour to a substrate such as cloth, paper, plastic, or leather in reasonably permanent. Dyes are classified according to their chemical structure, and are composed of group of atoms known as chrompohores, responsible for dye colour. These chromophore-containing centers are based on diverse functional groups, such as azo, anthraquinone, methine, nitro, arylmethane, carbonyl and others. In addition, electrons withdrawing or donating substituents so as to generate or intensify the color of the chromophores are denominated as auxochromes. The most common auxochromes are amine, carboxyl, sulfonate and hydroxyl.
It is estimated that over 10,000 different dyes and pigments are used industrially and over 7 x 105 tons of synthetic dyes are annually produced worldwide. In the textile industry, up to 200,000 tons of these dyes are lost to effluents every year during the dyeing and finishing operations, due to the inefficiency of the dyeing process. Most of the industries are treating waste water but some of the industries are sending the waste water directly into river which contains harmful chemical that affects the aquatic life.
It is, therefore, an important aim of industrial fundamental research to develop new technologies, not only to optimize conventional processes but also to solve respective problems by basically novel concepts. Hence, to optimize conventional processes the technology comes into existence which is Supercritical Fluid Dyeing techniques but not commercially used in all textile processing industries but one day this technique will be used commercially because it offers various advantages than conventional dyeing techniques.
What is Supercritical Fluid?
Supercritical fluid is one kind of fluid which is highly compressed gases which combine properties of gases and liquids in an intriguing manner. It is a substance which can be either liquid or gas, used in a state above the critical temperature and critical pressure where gases and liquids can coexist. It shows unique properties that are different from those of either gases or liquids under standard conditions.
A supercritical fluid has both the gaseous property of being able to penetrate anything, and the liquid property of being able to dissolve materials into their components. In addition, close to the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be “fine-tuned”. Carbon dioxide and water are the most commonly used supercritical fluids.
What is Supercritical Fluid Dyeing?
Here Carbon Dioxide is used as a supercritical fluid as carbon dioxide is readily available, cheap and is non-toxic and non-flammable. Above the temperature of 31.06 Degree Celsius and pressure of 72.8 atmosphere exhibit physical properties, which are intermediate between properties of liquid and gas. These are supercritical conditions and can be achieved by using commercially available equipment.
The main advantage of this process is that solvent can be easily turned into gas by simply releasing the pressure leaving no solvent residues and requires no evaporation orseparation. The low viscosity of supercritical fluids and the rather high diffusion properties of the dissolved molecules are especially promising aspects for dyeing processes. A supercritical dyeing fluid should easily dissolve solid dyestuffs and should penetrate even the smallest pores without the need of vigorous convection procedures.
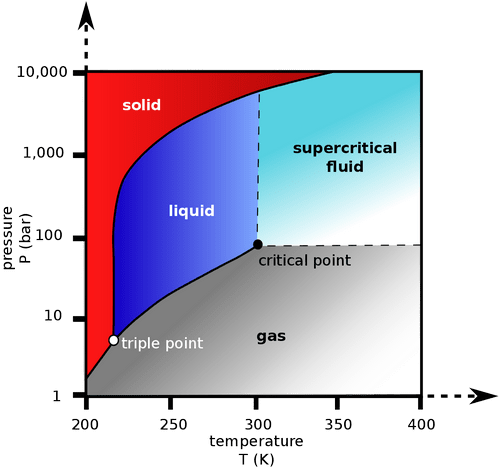
A supercritical fluid may be characterized best by referring to a phase diagram as shown for in Fig 2. A liquid can be converted to a supercritical fluid by increasing its temperature (and consequently its vapor pressure) and simultaneously increasing pressure. A closed system thus reaches critical values where no boundary between the liquid and gaseous state can be distinguished, i.e., the supercritical state.
Why Supercritical Dyeing is better than conventional dyeing technique?
- Waste water is not generated as water is not used while dyeing
- No damage to the fibre
- No need of dispersing or levelling agents
- Short time as compared to conventional dyeing
- Low dyes and chemical consumption
- Carbon Dioxide can be recycled easily hence no air pollution
- Drying is not required
- Low energy consumption
- No effluents
- Environment friendly process
What is the dis-advantages or limitations of Supercritical Fluid Dyeing technique?
- Initial investment is high
- Processing of long length fabric (continuous process) is not possible
- At present Supercritical fluid dyeing is only suitable for synthetic fibres
Conclusion
The dyeing technique of supercritical CO2 has been established successfully in recent years in practice. It can be expected that more textile dyehouses would acquire scf-CO2 dyeing machines. The rate-determining factor in the market currently seems to be how quickly the manufacturer can build and install their machines. Currently, the scf-CO2 dyeing is running only with polyester (PET). Since polyester represents the largest and most important fibre class, this is for the further growth of the technology with no real obstacle. This innovative technology of the scf-CO2 dyeing could therefore be a sustainable success, despite a long development time.
References
- Textile Research-Journal 1993,63 (3) 135-142
- https://www.fibre2fashion.com/industry-article/1678/super-critical-carbon-dioxide-assisted-dyeing
- https://en.wikipedia.org/wiki/Supercritical_fluid
